The leukapheresis procedure is a modified apheresis (dual needle)collection involving the removal of a healthy contributor’s whole blood and separation of white blood cells. Inherent in the procedure are increased risks to the donor related to the extended procedure duration, larger blood volume processed, and, in the case ofmobilized donations, inclusion of the G-CSF administration process.3
Identifying, recruiting, screening and managing white blood cell contributors is a multi-faceted process that is integral to collecting Leukopak products to satisfy the needs of researchers around the world. An organization with demonstrated experience inA Blood Center’s Unique Position Enabling Cellular Therapy Researchblood product collection and donor management is an ideal partner in mitigating these challenges and the increased risks that come with leukapheresis. Maintaining a healthy, engaged contributor bank with diverse characteristics, in tandem with navigating the highly regulated Leukopak collection environment, requires an organization with deep expertise in transfusion medicine and blood collection.
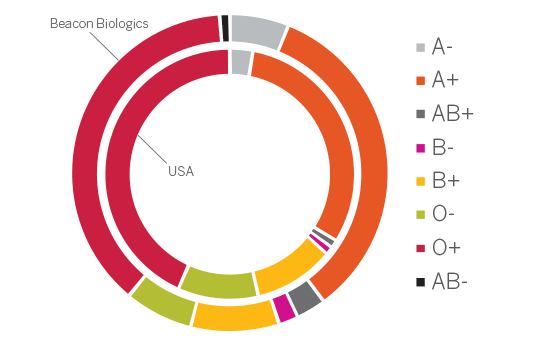
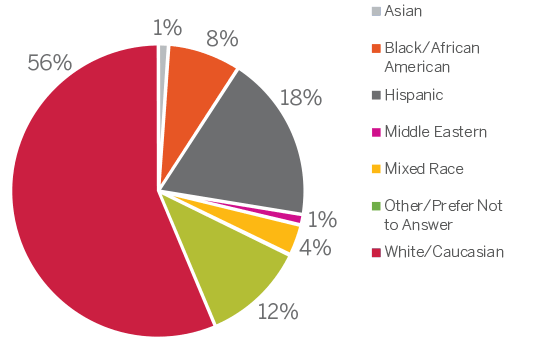
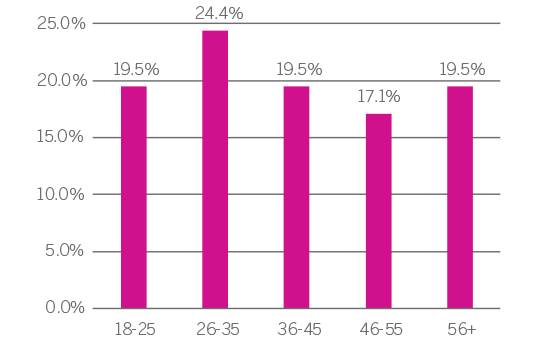
Due to this confluence of factors, blood centers are uniquely positioned to provide leukopaks for clinical research. The provision of biomaterials is an undeniably critical component for clinical research. Access toa diverse, well-characterized, recallable contributor base enables research to proceed without undue delay related to recruiting, identifying, screening and drawing new contributors. In a process with innumerable variables, a contributor bank operating under an IRB-approved protocol helps mitigate risk in early-stage trials by adhering to stringent contributor recruitment criteria, ensuring a safe procedure for the donor, and timely provision of products for researchers.
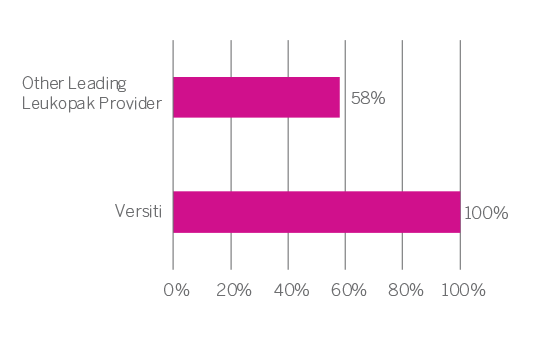
As a leading blood provider with 75 years of experience serving local communities through the provision of blood products for transfusion, Versiti performs over 115,000 apheresis procedures annually. In 2020, Versiti added the provision of leukopak products to support emerging cellular therapy research to its comprehensive suite of services. Our established leukopak contributor bank operates under an IRB-governed protocol,ensuring both the contributor experience and resulting blood products meet strict quality and regulatory standards. To ensure product quality, Versiti provides a certificate of analysis and independent review with every product, ensuring that each collected leukopak meets strict quality standards and study requirements.