Enabling In-Vitro Diagnostics
Your IVD Partner, From Bench to Bedside

Built on Diagnostic Excellence
Trusted by clinicians and researchers nationwide.
Expertise in Hematology & Rare Diseases
Access to richly characterized donors and complex phenotypes.
Tailored, Consultative Leadship
Integrated collaboration allows us to work as an extension of your team.
Starting Biospecimen Access
Leukopaks, buffy coats, plasma, and whole blood from targeted populations.
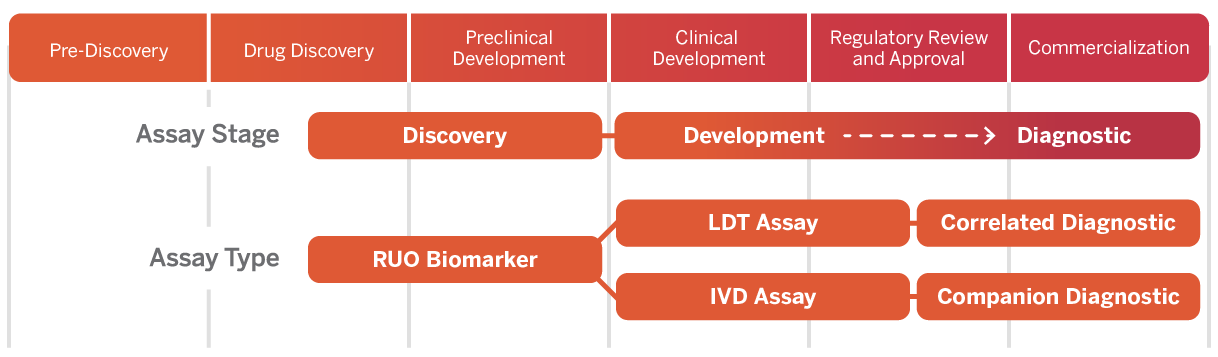
Comprehensive Clinical Trial Services and Capabilities
Explore the comprehensive suite of clinical trial services and capabilities offered by Versiti Clinical Trial Services, supporting sponsors from study design to regulatory filings and custom assay development.
Clinical Trial Management
From protocol management to regulatory compliance, our clinical trial management services ensure seamless, end-to-end study support you can rely on.
Explore Our Diagnostic Test Menu
Diagnostic laboratory services including HLA testing, donor testing, immunohematology reference lab testing, hematology testing and more.