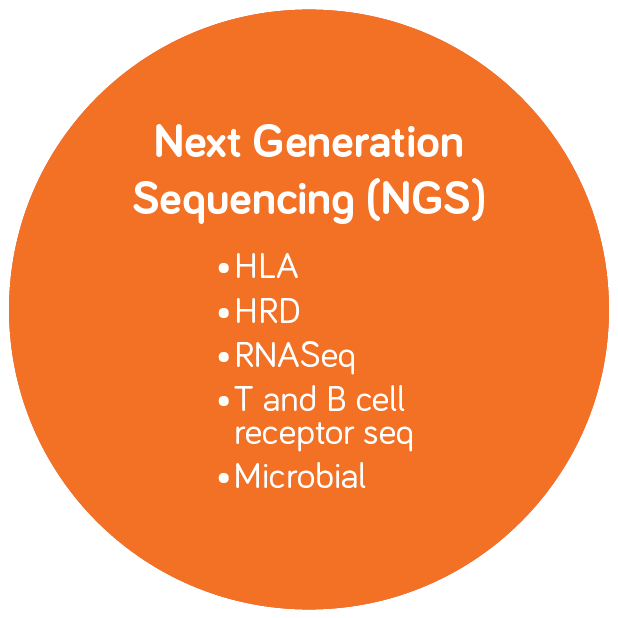
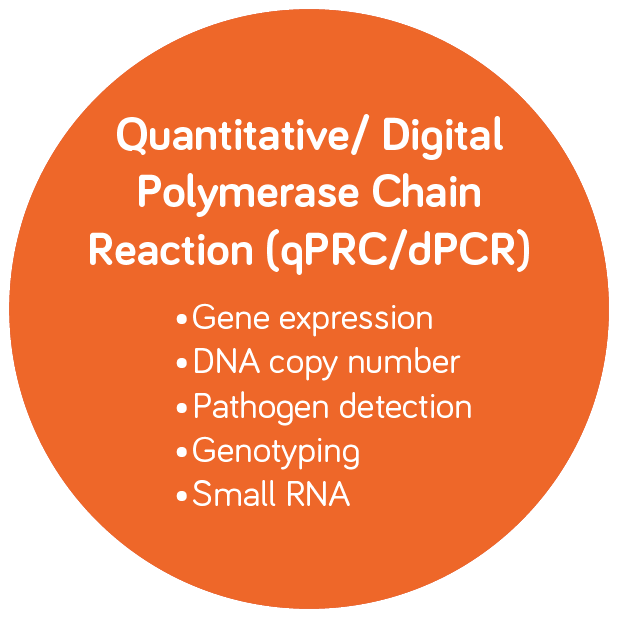
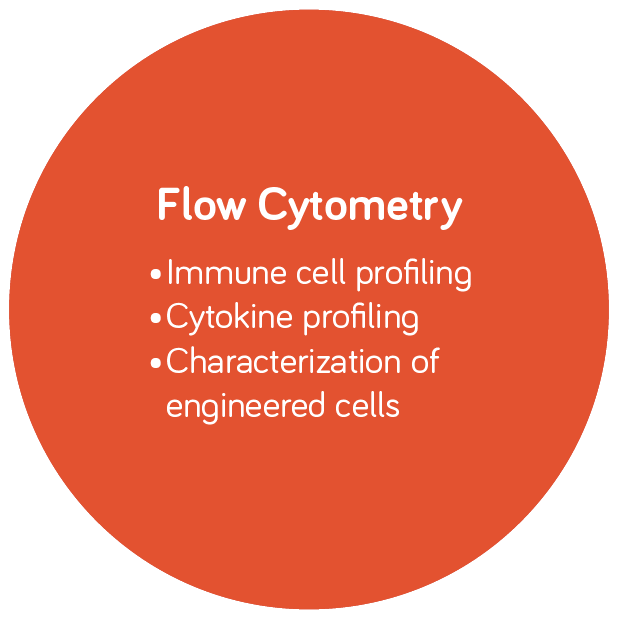
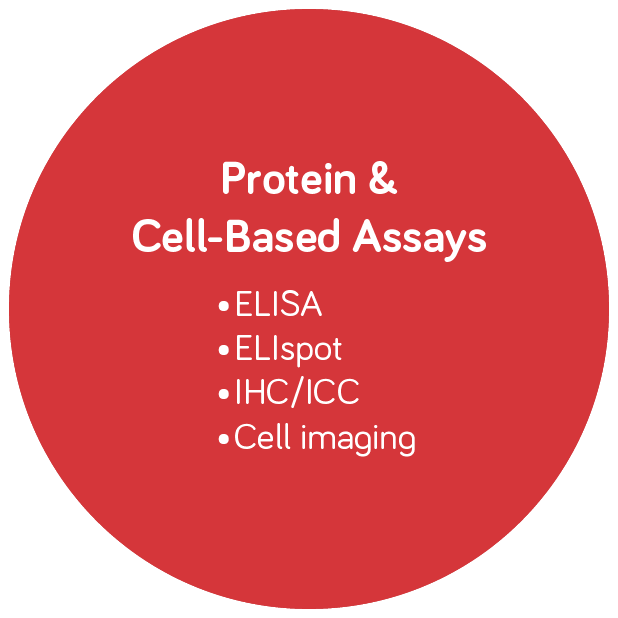
Molecular Diagnostics and Infectious Disease Testing
Advancing Precision Medicine Through Partnership and Innovation

Study Design & Site Selection Services
Optimize your Clinical Trial Study Design with expert site selection and monitoring services tailored to ensure trial success from start to finish.
Data & Project Management
At Versiti Clinical Trial Services, we provide dedicated project and data management teams to support your clinical trials from start to finish.
Collection Kits, Biologistics & Clinical Supplies
Versiti offers specimen collection kit design, clinical supplies, and full biologistics support—streamlining trials from site to shipment.
Quality & Regulatory Services
Versiti’s Quality and Regulatory services equip therapeutic developers with the expertise to navigate complex regulations and ensure compliance.