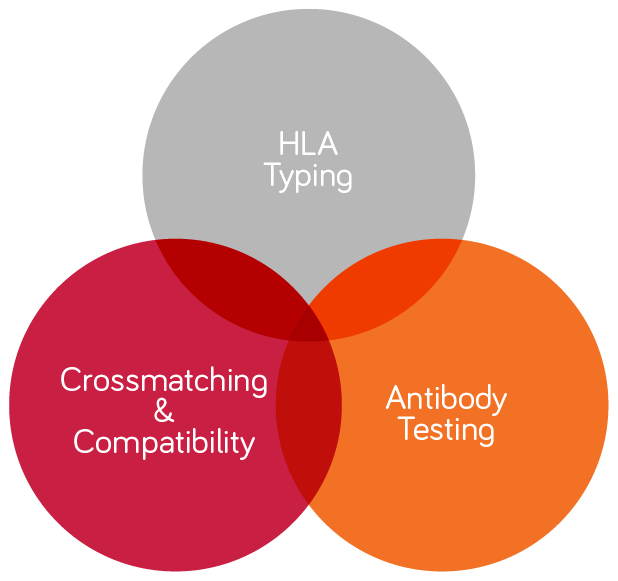
Biomarker, Assay Development & HLA Typing
Comprehensive Immunogenetics Solutions for Translational Research

Laboratory & Diagnostic Services
Versiti Clinical Trial Services provides central laboratory services, including sample testing, assay development, site management and more.
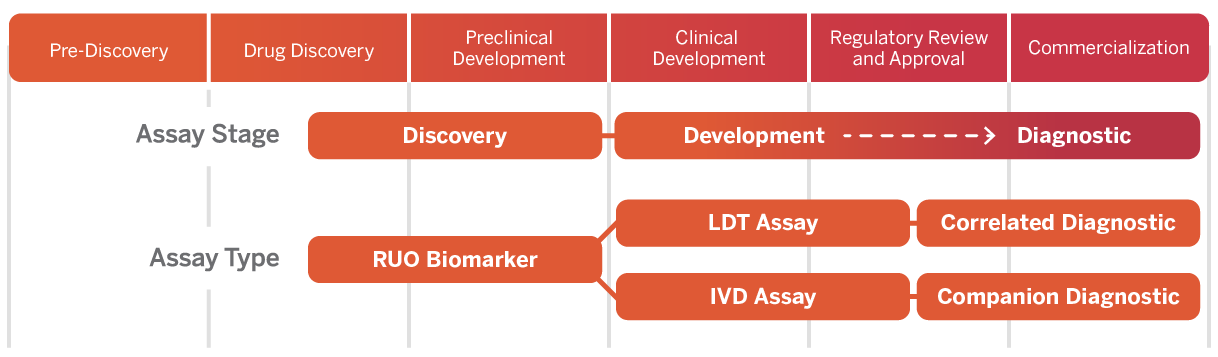
Companion Diagnostics
Companion Diagnostic (CDx) assay development to advance therapies with precise, on-time delivery of actionable data for your unique project.
Quality & Regulatory Services
Versiti’s Quality and Regulatory services equip therapeutic developers with the expertise to navigate complex regulations and ensure compliance.