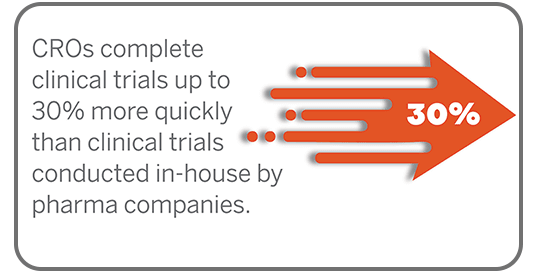
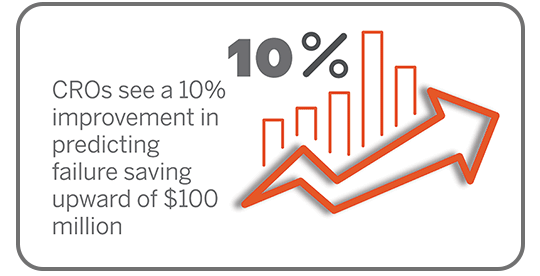
Clinical Trial Management
From protocol management to regulatory compliance, our clinical trial management services ensure seamless, end-to-end study support you can rely on.
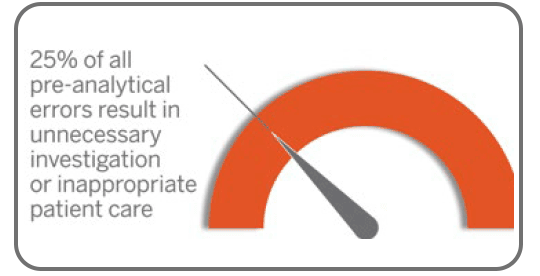
Laboratory & Diagnostic Services
Versiti Clinical Trial Services provides central laboratory services, including sample testing, assay development, site management and more.
Quality & Regulatory Services
Versiti’s Quality and Regulatory services equip therapeutic developers with the expertise to navigate complex regulations and ensure compliance.