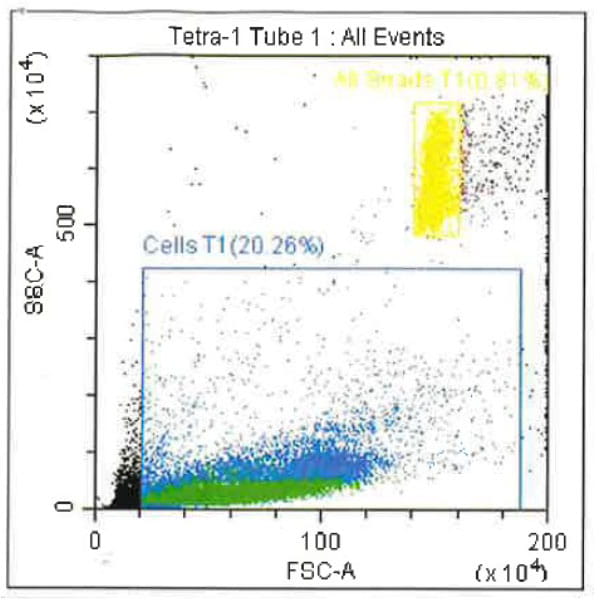
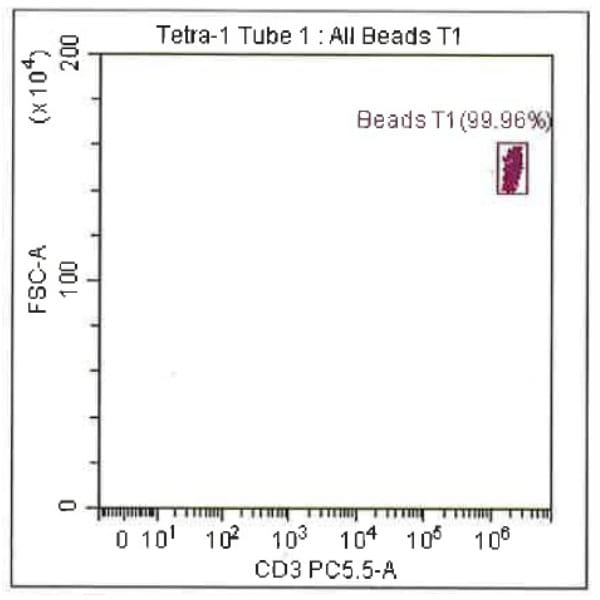
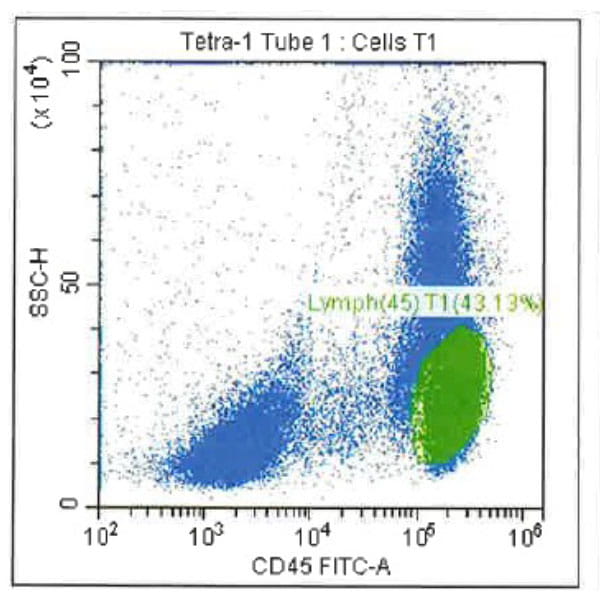
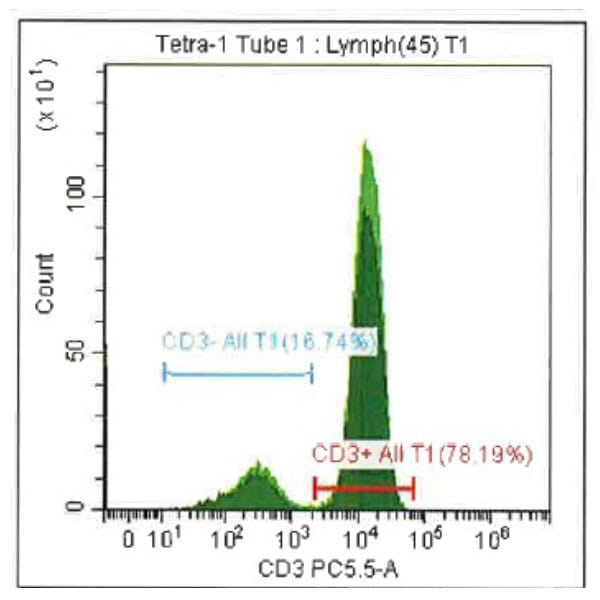
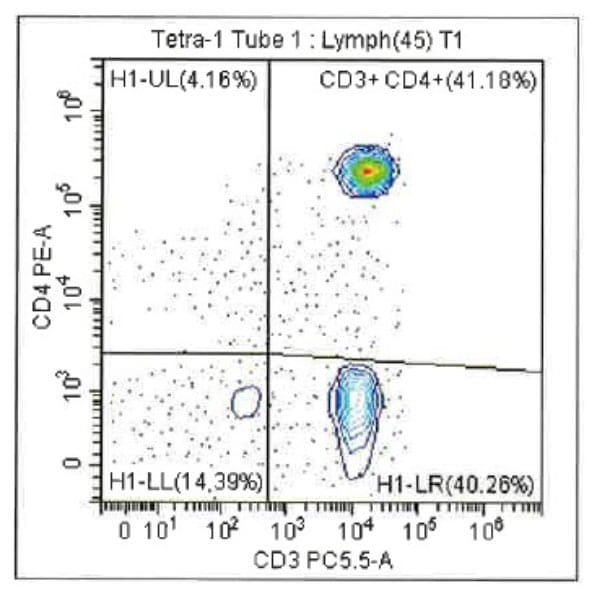
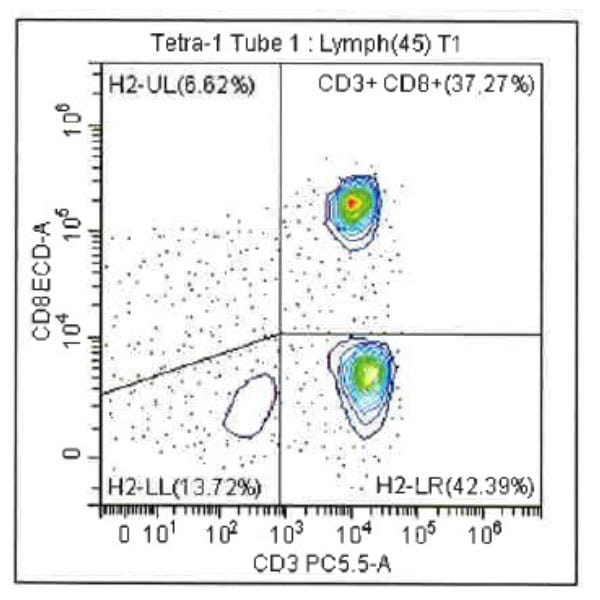
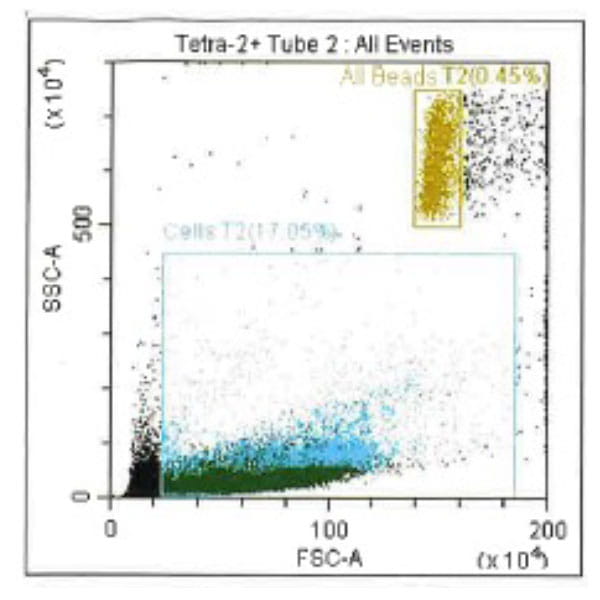
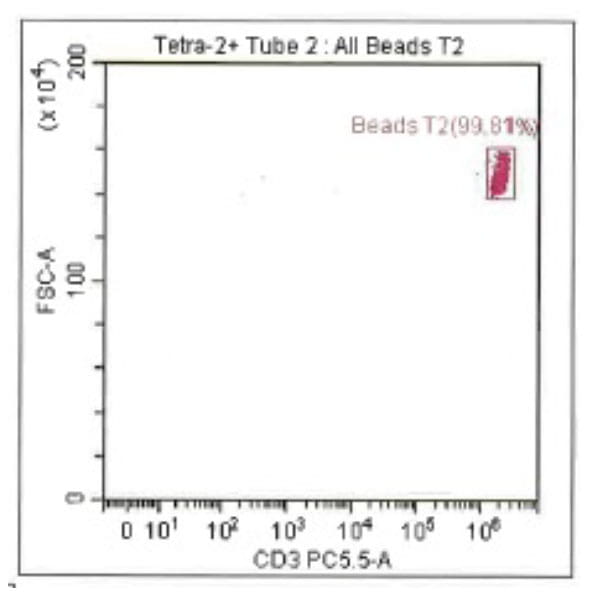
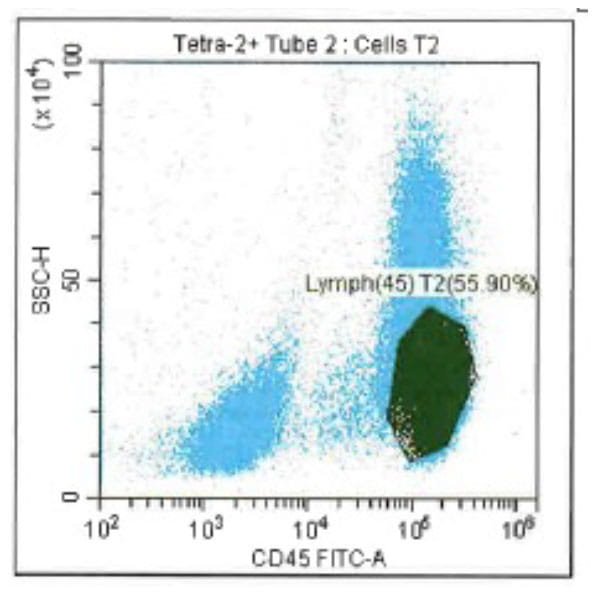
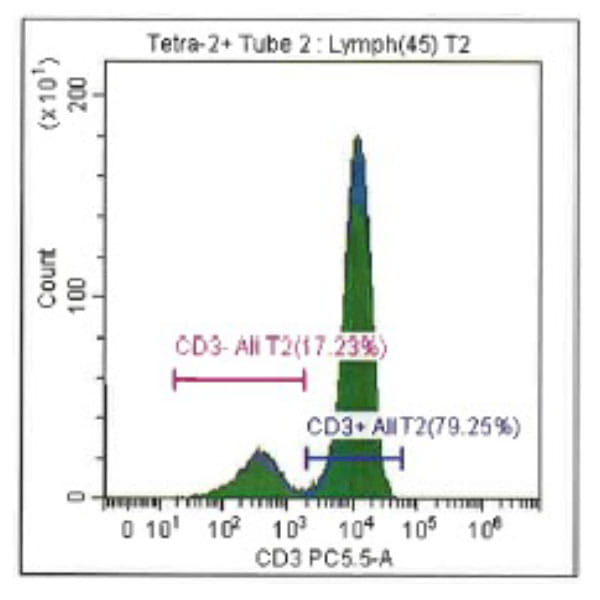
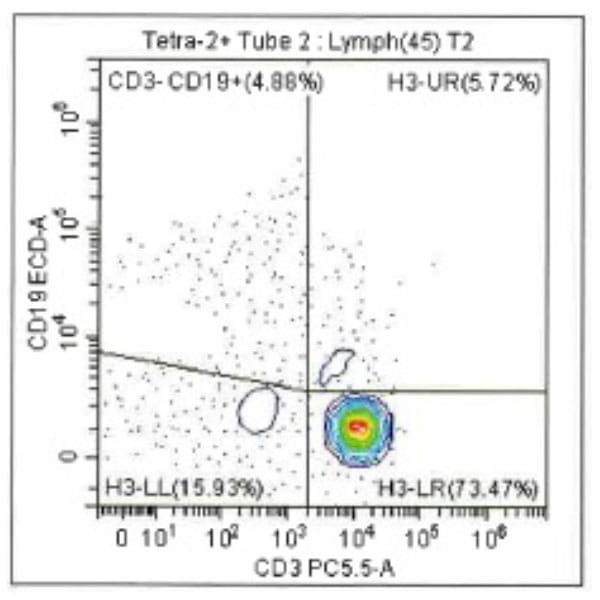
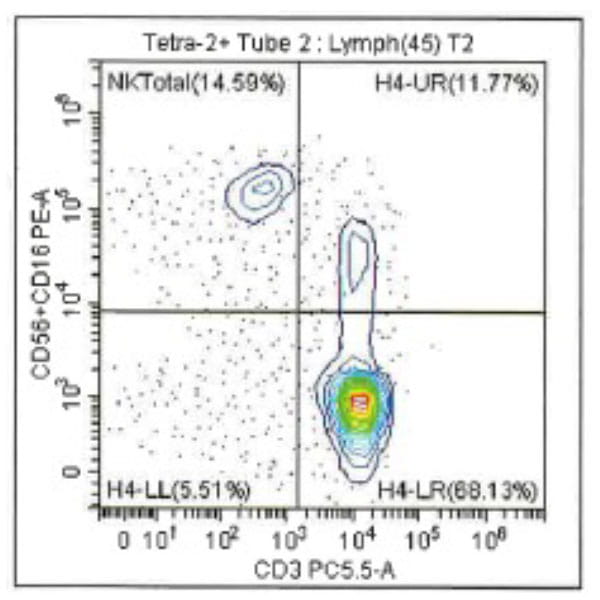
Leukopak products vary in cell counts due to several reasons, including execution of the leukapheresiscollection process and system utilized, donor characteristics, and whether the donor was able to providethe required blood volume to process the total number of cells required by the research protocol.
The leukopak collection process uses one single donor per leukopak product collected. The volume ofwhite blood cells in a single leukopak allows the researcher to perform necessary assays, validation andquality assurance.
As with any human-derived starting material, innate variability exists in leukapheresis collections donorto-donor, and even collection-to-collection from the same donor. Each donor’s cell characteristics andquantification may impact the types of cells collected during leukapheresis. To maximize cell quality orquantity, a commitment to staff education and robust training on leukapheresis procedures and donormanagement is essential.
Dedicated resources and training provided to staff on leukapheresis protocols and cell therapycollections increases collection efficiency to meet cell yield requirements for the researcher, as well asprovides for a positive donor experience. Protocols should be developed to assist with streamlining thecollection process for both the researcher and donor.
A comprehensive donor management strategy is needed to support a research project or study.Identifying, recruiting, screening and managing donors is integral to collecting leukopak products.Managing the health of a donor is important to prevent fatigue and reduced cell counts. To mitigatedonor challenges and help ensure consistency in production, having committed, recallable donorsassigned to a specific project ensures a consistent yield of cells to keep the progress running smoothlythroughout the duration of research. Adhering to stringent donor criteria, operating under an IRBapprovedprotocol ensures a safe leukapheresis procedure for the donor.